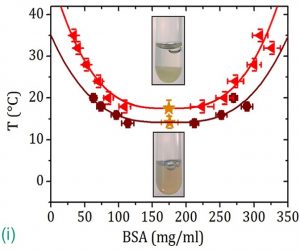
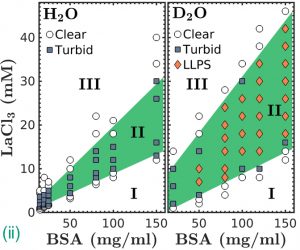
Protein condensation issues are related to a number of diseases: Alzheimer’s and sickle cell anaemia are associated with aberrant protein-protein interactions leading to the formation of fibrillar aggregates in the brain and in red blood cells respectively; droplets of concentrated protein formed due to altered protein-protein interactions result in liquid-liquid phase separation (LLPS) as found in eye cataracts [1]. It is known from colloid science that shortrange attractions between colloids can lead to LLPS. Coarse-grained soft matter approaches employing simple models from colloid theory are often successful in describing the main features underlying the behaviour of protein solutions, although proteins are highly complex and feature a broad variety of interactions. In experiments performed in our group, we introduce short-range attractions by adding the trivalent salt YCl3 to aqueous solutions of the negatively charged, globular protein bovine serum albumin (BSA). LLPS is then induced by the Y3+ cations bridging the BSA molecules. We recently showed this LLPS to be inducible by a temperature increase, a rather unusual observation for globular proteins, and presented a picture of the underlying mechanism [2] (Fig. (i)).
Using ultra-small angle X-ray scattering, we observed the formation of a reversible “arrested state” in concentrated BSA-YCl3 systems at temperatures below the denaturation temperature of BSA. This state is similar to a gel of concentrated protein [3]. As protein-based drugs are increasingly successful, but the search for non-invasive routes of administration is challenging [4], these findings suggest a possible pharmaceutical application: concentrated native proteins in gel-like states could be promising for non-parenteral formulations.